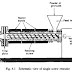
Acetal polymers are formed from the polymerization of formaldehyde.
They are also known by the name polyoxymethylenes (POM). Polymers
prepared from formaldehyde were studied by Staudinger in the 1920s,
but thermally stable materials were not introduced until the 1950s
when DuPont developed Delrin.1 Homopolymers are prepared from
very pure formaldehyde by anionic polymerization, as shown in Fig.
1.4. Amines and the soluble salts of alkali metals catalyze the reaction.2
The polymer formed is insoluble and is removed as the reaction proceeds.
Thermal degradation of the acetal resin occurs by unzipping
with the release of formaldhyde. The thermal stability of the polymer
is increased by esterification of the hydroxyl ends with acetic anhydride.
An alternative method to improve the thermal stability is copoly
merization with a second monomer such as ethylene oxide. The copolymer
is prepared by cationic methods.3 This was developed by Celanese
and marketed under the tradename Celcon. Hostaform is another
copolymer marketed by Hoescht. The presence of the second monomer
reduces the tendency for the polymer to degrade by unzipping.4
There are four processes for the thermal degradation of acetal
resins. The first is thermal or base-catalyzed depolymerization from
the chain, resulting in the release of formaldehyde. End capping the
polymer chain will reduce this tendency. The second is oxidative
attack at random positions, again leading to depolymerization. The
use of antioxidants will reduce this degradation mechanism.
Copolymerization is also helpful. The third mechanism is cleavage of
the acetal linkage by acids. It is, therefore, important not to process
acetals in equipment used for polyvinyl chloride (PVC), unless it has
been cleaned, due to the possible presence of traces of HCl. The fourth
degradation mechanism is thermal depolymerization at temperatures
above 270°C. It is important that processing temperatures remain
below this temperature to avoid degradation of the polymer.5
Acetals are highly crystalline, typically 75% crystalline, with a melting
point of 180°C.6 Compared to polyethylene (PE), the chains pack
closer together because of the shorter C O bond. As a result, the polymer
has a higher melting point. It is also harder than PE. The high
degree of crystallinity imparts good solvent resistance to acetal polymers.
The polymer is essentially linear with molecular weights (Mn) in
the range of 20,000 to 110,000.7
Acetal resins are strong and stiff thermoplastics with good fatigue
properties and dimensional stability. They also have a low coefficient
of friction and good heat resistance.8 Acetal resins are considered similar
to nylons, but are better in fatigue, creep, stiffness, and water
resistance.9 Acetal resins do not, however, have the creep resistance of
polycarbonate. As mentioned previously, acetal resins have excellent
solvent resistance with no organic solvents found below 70°C, however,
swelling may occur in some solvents. Acetal resins are susceptible
to strong acids and alkalis, as well as oxidizing agents. Although the
C O bond is polar, it is balanced and much less polar than the carbonyl
group present in nylon. As a result, acetal resins have relatively
low water absorption. The small amount of moisture absorbed may
cause swelling and dimensional changes, but will not degrade the polymer
by hydrolysis.10 The effects of moisture are considerably less dramatic
than for nylon polymers. Ultraviolet light may cause
degradation, which can be reduced by the addition of carbon black. The
copolymers generally have similar properties, but the homopolymer
may have slightly better mechanical properties, and higher melting
point, but poorer thermal stability and poorer alkali resistance.11
Along with both homopolymers and copolymers, there are also filled
materials (glass, fluoropolymer, aramid fiber, and other fillers), toughened
grades, and ultraviolet (UV) stabilized grades.12 Blends of acetal
with polyurethane elastomers show improved toughness and are available
commercially.
Acetal resins are available for injection molding, blow molding, and
extrusion. During processing it is important to avoid overheating or the
production of formaldehyde may cause serious pressure buildup. The
polymer should be purged from the machine before shutdown to avoid
excessive heating during startup.13 Acetal resins should be stored in a
dry place. The apparent viscosity of acetal resins is less dependent on
shear stress and temperature than polyolefins, but the melt has low
elasticity and melt strength. The low melt strength is a problem for
blow molding applications. For blow molding applications, copolymers
with branched structures are available. Crystallization occurs rapidly
with postmold shrinkage complete within 48 h of molding. Because of
the rapid crystallization it is difficult to obtain clear films.14
The market demand for acetal resins in the United States and
Canada was 368 million pounds in 1997.15 Applications for acetal
resins include gears, rollers, plumbing components, pump parts, fan
blades, blow-molded aerosol containers, and molded sprockets and
chains. They are often used as direct replacements for metal. Most of
the acetal resins are processed by injection molding, with the remainder
used in extruded sheet and rod. Their low coefficient of friction
make acetal resins good for bearings.16
Modern
Plastics
Handbook
Modern Plastics
and
Charles A. Harper Editor in Chief
Technology Seminars, Inc.
Lutherville, Maryland
McGraw-Hill
New York San Francisco Washington, D.C. Auckland Bogotá
Caracas Lisbon London Madrid Mexico City Milan
Montreal New Delhi San Juan Singapore
Sydney Tokyo Toronto